Anytime the Corn Belt experiences large amounts of rain it raises questions about possible N loss and the need for supplemental applications. Understanding the factors that influence nitrogen loss will help growers determine their specific situation and help them make more informed decisions.
Understanding Nitrogen Forms
One of the factors that influences nitrogen loss is the form present in the soil. Nitrogen exists in many forms and transforms from one form to another in a process known as the nitrogen cycle. Nitrogen is primarily introduced to the soil either through applied fertilizer, or the breaking down of crop residue and soil organic matter.
Nitrogen fertilizers can be divided into two categories: Ammonium fertilizers (anhydrous ammonia and ammonium sulfate) and urea-containing fertilizers (Urea, 28%, 32%, and manure). Ammonium fertilizers are applied in the ammonium (NH4+) form. Urea-containing fertilizers are often a combination of ammonium and nitrate (NO3-). If the nitrogen stays in the ammonium form, loss would not occur because it attaches to the soil and is immobile. However, ammonium will naturally be converted to nitrate through a process called nitrification. Nitrogen in the nitrate form can then be lost by leaching or denitrification.
The Nitrogen Cycle
The conversion of ammonium to nitrate (nitrification) and the conversion of nitrate to N gases (denitrification or volatilization) are processes that depend heavily on the environment. Understanding the key factors for each process will help you determine the potential for N loss.
Nitrification is greatly influenced by soil temperature. The process is faster with warm soils, while slow to nonexistent in cold soils. This is the reason it’s recommended to wait until soils cool to 50°F in the fall before applying any ammonium fertilizers. If soil temperatures stay cool, the nitrogen will stay in ammonium form and be immobile. If soil temperatures warm above 50°F, nitrification will begin to take place and the ammonium will start to be converted by microbes to the more mobile nitrate form. When urea or urea-containing fertilizers are applied in the spring, they are usually converted to nitrate more rapidly than ammonium applied in either the fall or spring. Conversion to nitrate does not equal loss, but rather that it is more susceptible to loss.
Leaching can occur once nitrogen has been converted to nitrate. Nitrate is water soluble and will not attach to the soil, so as water passes through the soil, excess nitrate can move below the root zone in certain conditions. In general, leaching losses are more likely in sandy soils with low water holding capacity where water moves through the profile quickly. Another factor that influences the amount of leaching is subsoil moisture. If subsoil moisture levels are not recharged, water is more likely to be held in the soil, therefore reducing the chances of leaching.
Denitrification only occurs when soils are saturated and in an anaerobic environment. This process is heavily influenced by temperature as well. Figure 1 shows the amount of denitrification expected at the given soil temperatures when soils are saturated for an extended period.
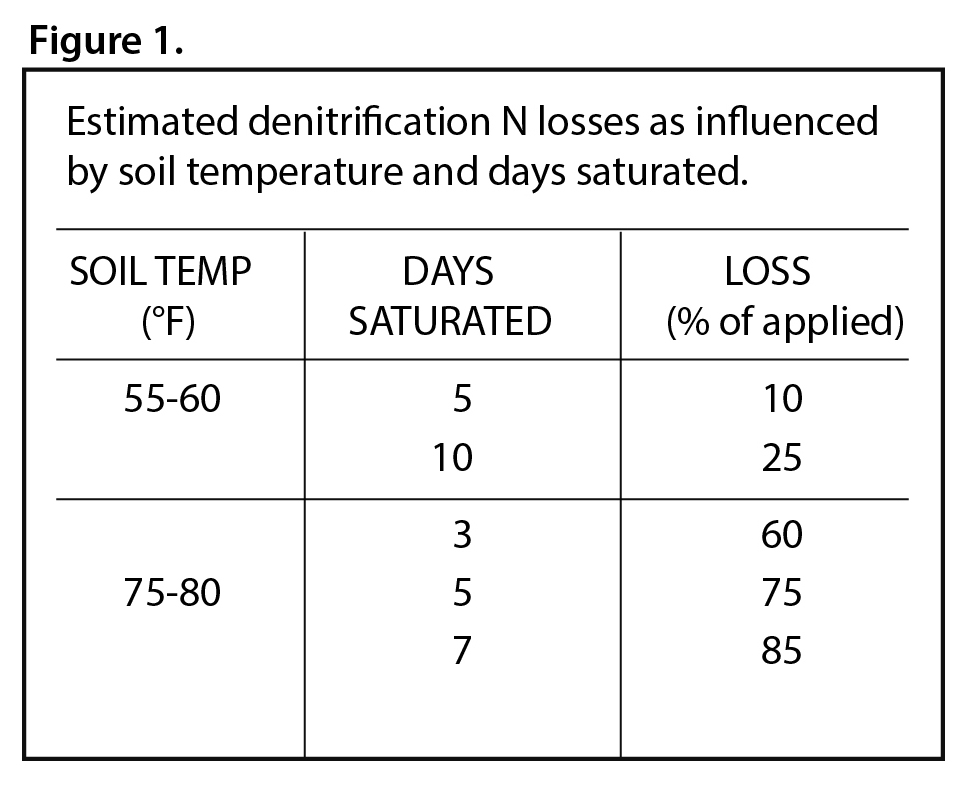
If soils are still cool when the extended saturation occurs, the rate of denitrification is greatly reduced. Denitrification, in general, is more likely on fine textured, poorly drained soils where saturation is maintained for several days. When soils are saturated early in the spring before soil temperatures rise, the risk of denitrification is reduced.
Volatilization occurs when nitrogen is in the organic form of urea, most commonly from animal manure or urea fertilizers. When this happens, the nitrogen is changed to ammonia gas (NH3) and lost into the atmosphere. It is most likely to take place when soils are warm and moist, and the source of urea is near the surface. Applying urea fertilizers when soil and air temperatures are cool and incorporating the fertilizer into the soil will reduce risk of volatilization.
Preventing Nitrogen Loss
There are steps that can be taken throughout the year to minimize nitrogen loss. When to implement these depend on what form of nitrogen you are applying, when it is applied, and the environment in which it is applied.
There are products available known as nitrification inhibitors which slow the nitrification process allowing nitrogen to stay in the immobile ammonium form later into the year. These products can be used in fall and spring applications to delay nitrification until later in the spring when the crop needs it, reducing the amount of nitrate that could possibly leach through the soil.
When applying ammonium in the fall, make sure to wait until soil temperatures fall below 50°F so that nitrification does not occur. Using a nitrification inhibitor will also help delay the nitrification process, preventing nitrogen loss.
In the spring, when applying urea-containing fertilizers you can reduce volatilization by incorporating it into the soil. Urea will convert to nitrate much faster than ammonium fertilizer. Therefore, using urease inhibitors and avoiding application of the fertilizer too long before your projected planting date will reduce the amount of nitrogen lost.
Nitrogen can be one of the leading limiting factors for a corn crop.
Making sure that you have enough N present to reach your yield goal is important. You first must set a yield goal to determine how much N will be required to meet that goal. Next you must determine how much N is available or will become available in the soil from the previous year’s crop and crop residue. There are differing methods and formulas for determining the total amount of N to apply, but all are based off the same principles.
Rules of thumb to determine N loss:
- Above average rainfall during the months of March-June
- Iowa State University has determined that more than 16 inches of rainfall during this time justifies an extra 20-50 lbs of additional N above normal application rates.
- Denitrification losses range from 2% (55-60°F air temperature) to +5% (65°F+ air temperature) per day
- This can be a significant loss pathway during springs where ponding is present.
One proven way to determine the amount of N present in your fields is to collect a soil sample and analyze it for NO3 and NH4. If you discover, or suspect, that there is not as much carryover nitrogen available, it would be wise to consider a supplemental application of N at some time during the growing season. This will help replace N lost during periods of above average rainfall.
Summary
Nitrogen is one of the most yield-limiting inputs in most crop production systems, however we can control when and how we apply our N fertilizers. Often times this is a balancing act between profitability and maximizing productivity. Nitrogen is one of the costliest inputs in corn production, so it’s a decision that demands careful attention. A shortfall can turn into a costly mistake.
